Gastric cancer
is the fourth most commonly diagnosed cancer and the second most common cause
of cancer related death worldwide. Signet-ring cell cancer is a subtype of
adenocarcinoma, which is the most common type of cancer arising from the
stomach. It has a distinct appearance under the microscope as a result of the
clear mucin it produces either inside or outside the cells. The natural course
of patients with signet ring cell carcinoma of the stomach remains poorly
understood while assumptions have been made to distinguish it from other types
of gastric cancer.
INTRODUCTION
Gastric cancer
is the fourth most commonly diagnosed cancer and the second most common cause
of cancer related death worldwide. Adenocarcinoma is the most common type of
cancer arising from the stomach. The incidence of adenocarcinoma of the stomach
is declining worldwide. Today, gastric cancer is still the seventh most common
cause of cancer-related death in the United States and the prognosis of
advanced gastric cancer remains poor. Signet-ring cell cancer is a subtype of
adenocarcinoma, which is the most common type of cancer arising from the
stomach. The natural course of patients with signet ring cell carcinoma of the
stomach remains poorly understood while assumptions have been made to
distinguish it from other types of gastric cancer. 1-10 It has a distinct appearance under the
microscope as a result of the clear mucin it produces either inside or outside
the cells. In most series, this cancer
has a poor prognosis.8 Signet ring cell carcinoma is a descriptive
term used to denote a form of mucin-secreting adenocarcioma whose component
cells retain abundant intracytoplasmic mucin, pushing their nuclei to one side,
creating
a classical signet ring cell appearance thereby giving the cells their characteristic histological appearance.1-6,8
Signet
ring cell carcinoma is poorly cohesive carcinomas and often composed of a
mixture of signet ring cells and non-signet ring cells. Those tumor cells can
form irregular microtrebaculae or lace-like abortive glands, often accompanied
by marked desmoplasia in the gastric wall and with a grossly depressed or
ulcerated surface.1,2,3-9 The overall survival rate of patients
with signet ring cell carcinoma was worse than that of patients with other
types of gastric cancer.1,4
Gastric cancer is the fourth most commonly
diagnosed cancer and the second most common cause of cancer related death
worldwide. Although the incidence of gastric cancer has gradually decreased
over the last half century, cancer at proximal stomach is on the rise. Today,
gastric cancer is still the seventh most common cause of cancer-related death
in the United States and the prognosis of advanced gastric cancer remains poor.
Gastric carcinogenesis is a multistep and multifactorial process.1,10
Histologically, gastric carcinoma demonstrates marked heterogeneity at both
architectural and cytologic level, often with co-existence of several
histologic elements. Gastric cancer incidence rates for men are approximately
twice those of women in all populations for persons over 50 years of age, but
the male:female incidence ratio is 1 or less at younger ages. The age-related
variation in the male:female incidence ratio suggests that host factors may
influence the level of risk of acquiring this cancer.2,3
Gastric carcinoma is clinically classified as
early or advanced stage to help determine appropriate intervention, and
histologically into subtypes based on major morphologic component. Early
gastric carcinoma is defined as invasive carcinoma confined to mucosa and or
submucosa, with or without lymph node metastases, irrespective of the tumor
size. Most early gastric carcinomas are small, measuring 2 to 5 cm in size, and
often located at lesser curvature around angularis. Some early gastric
carcinoma can be multifocal, often indicative of a worse prognosis.1,10
Advances gastric
carcinoma which invades into muscularis propria or beyond carries a much worse
prognosis, with a 5 years survival rate at about 60% or less.10 In this case, the tumor cells have been
extension to the muscularis propria, and surgical margins still found tumor
cells. The gross appearance of advanced gastric
carcinomas can be exophytic, ulcerated, infiltrative or combined. Based on Borrmann’s
classification, the gross appearance of advanced gastric carcinomas can be
divided into type I for polypoid growth, type II for fungating growth, type III
for ulcerating growth, and type IV for diffusely infiltrating growth which is
also referred to as linitis plastica in signet ring cell carcinoma when most of
gastric wall is involved by infiltrating tumor cells. 3,4,7,10 Histologically, advanced gastric
carcinoma often demonstrates marked architectural and cytological
heterogeneity, with several co-existing histologic growth patterns. The
distinction between early and advanced gastric carcinoma before resection is
clinically important because it helps decide if a neoadjuvant (pre-operative)
therapy which has shown to improve disease free survival and overall
survival is warranted. While the
macroscopic appearance is informative, the most accurate pre-operative staging
information is generally obtained with endoscopic ultrasonography (EUS) and
computer tomography (CT).7,10
Over the past
half century the histologic classification of gastric carcinoma has been
largely based on Lauren’s criteria (intestinal type and diffuse type), in
dominant elements of other histologic patterns.1-10 While the
intestinal type of gastric cancer is often related to environmental factors
such as Helicobacter pylori infection, diet, and life style. The diffuse type
is more often associated with genetic abnormalities. The intestinal type of gastric cancer is thought to arise from the
metaplastic epithelium (Lauren 1965), whereas signet ring cell carcinoma in the
diffuse type is thought to arise from the mucosa that is not metaplastic and is
confined to the glandular neck region in a proliferating zone (Carneiro et al,
1992; Kitamura et al. 1996).4 Signet-ring cell carcinomas are
infiltrative; the number of malignant cells is comparatively small and
desmoplasia may be prominent. Signet ring carcinomas are tumors in
which >50% of the mass consists of isolated or small groups of malignant cells containing
intracytoplasmic mucin that compresses the nucleus to the periphery of the
cell membranes. The classic signet ring cells are most numerous in the
superficial portions of the tumor where they are stacked in a layered fashion. Superficially, cells lie scattered in
the lamina propria, widening the distances between the pits and glands. The tumour cells have five morphologies: (1)
Nuclei push against cell membranes creating a classical signet ring cell
appearance due to an expanded, globoid, optically clear cytoplasm. These
contain acid mucin and stain with Alcian blue at pH 2.5; (2) other diffuse carcinomas
contain cells with central nuclei resembling histiocytes, and show little or no
mitotic activity; (3) small, deeply eosinophilic cells with prominent, but
minute, cytoplasmic granules containing neutral mucin; (4) small cells with
little or no mucin, and (5) anaplastic cells with little or no mucin. These
cell types intermingle with one another and constitute varying tumour
proportions. Signet-ring cell tumours may also form lacy or delicate trabecular
glandular patterns and they may display a zonal or solid arrangement.1-6
Signet ring
cancers usually exhibit the infiltrating growth pattern and desmoplasia that is
called diffuse cancer in the Lauren classification. Infiltrating Borrmann type
IV carcinomas are usually signet ring tumors. The tumor cells in the deeper
portions of the stomach wall have less cytoplasmic mucin and more centralized
nuclei. They may be so widely dispersed through the stroma that they may be
difficult to detect in routine H&E - stained preparations. Some form of
mucin stain (periodic acid Schiff, Alcian blue, mucicarmine) or
immunohistochemical staining with cytokeratin antibodies may be used to detect
these cells.1-6 Special stains, including mucin stains (PAS, mucicarmine, or
Alcian blue) or immunohistochemical staining with antibodies to cytokeratin,
help detect sparsely dispersed tumour cells in the stroma. Cytokeratin
immunostains detect a greater percentage of neoplastic cells than do mucin
stains. Several conditions mimic signet-ring cell carcinoma including signet-ring
lymphoma, lamina propria muciphages, xanthomas and detached or dying cells
associated with gastritis.1,2
The clinicopathological characteristics of signet ring cell
carcinoma of the stomach are known to differ from that of other types of
gastric cancer. Some have reported a higher rate of forming multiple gastric
cancers if the primary lesion is signet ring cell carcinoma. Early gastric
cancer with signet ring cell histology tends to be superficial and large,
leading to earlier diagnosis than gastic cancer of other histological types.
The superficial spreading nature may be helpful for early detection, because it
has less chance of being missed during endoscopy.7,10
In addition to a superficial spreading nature, the formation
of satelite lesions may be a significant characteristic of signet ring cell
carcinoma. Despite the advancement in diagnostic tools, a proportion of gastric
cancers often remain undetected. Some reports assert that early signet ring
cell carcinoma of the stomach tends to spread in the mucosal and submucosal
layers continously or discontinously. This may explain the prevalence of
multiple gastric cancers in the signet ring cell carcinoma. As an early gastric
cancer, signet ring cell carcinoma is known to have a tendency to form multiple
lesions. However, once the disease evolves into advanced gastric cancer, the
diffusely infiltrating characteristics of signet ring cell carcinoma may be
associated with a poor prognosis by involving the entire stomach, resulting in
what is known as linitis plastica. Advancement in diagnostic tools along with
signet ring cell carcinomas tendency to spread superficially may have resulted
in an apparently slow progression of this cancer. Although the results are not
consistent, some reports discuss that, as an early gastric cancer, signet ring
cell carcinoma has a favorable prognosis with less lymph node metastasis.7,8
When it occurs at the antropyloric region with
serosal involvement, the carcinoma tends to have lymphovascular invasion and
lymph node metastasis. Because signet ring cell and other poorly cohesive
carcinomas at antroplyoric region have a propensity to invade duodenum via
submucosal and subserosal routes including subserosal and submucosal lymphatic
spaces, special attention needs to be paid to those routes when a distal margin
frozen section is requested at the time of surgical resection. In this case,
tumor locate is unknown but the tumor cells have been infiltrated the
muscularis serosa. One important differential diagnosis of neoplastic signet ring
cells in gastric mucosa is benign pseudo-signet ring cells which can remarkably
mimic signet ring cell carcinoma. Those pseudo-signet ring cells sometimes can
demonstrate cytological atypia, even with mitoses. However, those pseudo-signet
ring cells do not reveal invasive pattern with reticulin stain which highlights
pseudo-signet ring cells confined within basement membrane with intact acinar
architecture.1,2,3,-9
There
were demographic differences between the two tumor types as well. In patients
with intestinal-type carcinoma, 65% were men and the average age was 55.4
years; in diffuse-type gastric carcinoma, 54% were men and the average age was
47.7 years. Approximately 60% of gastric cancers in high-risk
populations, are most common in the antrum. 1,2,4-6 The reasons for
age variation remain unknown; possibilities include genetic heterogeneity
between different CDH-1 mutations, other gene-gene interactions, or
environmental factors.7 The diffuse phenotype in gastric cancer
(hereditary and sporadic) is related to reduced E- adherin expression. It has
been suggested that loss of E-cadherin is the fundamental defect in diffuse
type gastric carcinoma, and provides an explanation for the observed
morphological phenotype: discohesive cells with loss of polarity and gland
architecture. In contrast, gland architecture is preserved in the intestinal
type of stomach cancer where loss of E-cadherin expression is not a feature.7
Diffuse carcinomas consist of
discohesive cells that penetrate through the stomach wall as individual cells
embedded in a desmoplastic stroma. As compared to the cells in intestinal-type carcinomas,
diffuse carcinoma cells were smaller, more uniform in overall shape and in
nuclear size, and had less mitotic activity. Epithelial structures formed by these
cells were more abortive, with only rare lumen formation. To the naked eye,
diffuse carcinomas did not form well-defined masses in many cases, and
microscopically showed extensive infiltration of the mucosa without associated
ulceration. Thirtyone per cent of these tumors were described as polypoid or
fungating; 43% were described as having a linitis plastica growth pattern. They appear
as layered signet ring cells in the superficial portions of the mucosa, but
signet ring cells are less numerous in the deeper portions of the wall, where
more pleomorphic cells predominate. Cell cycle markers may fail to label
superficial signet ring calls, in contrast to the discohesive cells that
infiltrate deeper into the gastric wall. A few inconspicuous, abortive glands
may be present. The desmoplastic reaction that accompanies diffuse cancer may
be more conspicuous than the cancer cells that elicit it.1,2,5
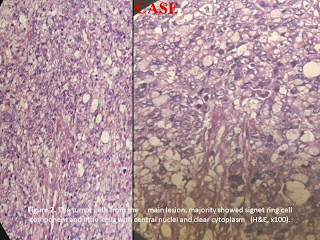
Molecular pathology in gastric carcinoma. An accumulation of genetic and molecular abnormalities occurs during gastric carcinogenesis, including activation of oncogenes, overexpression of growth factors/receptors, inactivation of tumor suppression genes, DNA repair genes and cell adhesion molecules, loss of heterogeneity and point mutations of tumor suppressor genes, and silencing of tumor suppressors by CpG island methylation and alterations in cell cycle regulatory genes. Inherited factors interact with environmental hazards to increase gastric cancer risk in two ways: (a) germline mutations account for well-defined, but infrequent, familial cancer syndromes; and (b) polymorphisms in genes that govern cell cycling or enzymes that catalyze carcinogen detoxification may also influence gastric cancer risk. Other types of changes occur in sporadic tumors.2,10Inherited gastric cancer predisposition syndromes account for approximately 10% of gastric cancers. The genetic events are known for some but not all of the predispositions. The hereditary nonpolyposis colon cancer syndrome (HNPCC) is an example of the interaction of environmental hazards with germline mutations. Persons with this autosomal dominant condition are at increased risk of developing cancer at sites other than the colon, including the stomach. HNPCC is due to a germline mutation in mismatch repair genes.1-3,5,10 There is some molecular overlap between the two types of gastric carcinoma; for example, mutations in the tumor suppressor gene TP53 are seen in both types of gastric carcinoma, as is silencing (by hypermethylation) of the mismatch repair gene MGMT. Defects in the CDH1 gene are almost exclusive to diffuse-type carcinomas. The CDH1 gene codes for the e-cadherin protein, which is important in cell–cell adhesion. Loss of expression of this protein correlates with loss of cohesion, leading to tumors that diffusely infiltrate as single or small groups of cells, often with intracellular accumulation of mucin giving a signet-ring appearance to the individual cells. This phenotype is mirrored by infiltrating lobular carcinoma of the breast. Most gastric carcinomas occur sporadically; only About 10% of gastric carcinomas show familial clustering but only approximately 1-3% of gastric carcinomas arise from inherited gastric cancer predisposition syndromes, such as hereditary diffuse gastric carcinoma (HDGC), familial adenomatous polyposis, hereditary nonpolyposis colorectal carcinoma (or Lynch syndrome), juvenile polyposis syndrome, Peutz-Jeghers syndrome, Li-Fraumeni syndrome and gastric hyperplastic polyposis. HDGC is an autosomal dominant disorder with high penetrance. Approximately 30% of individuals with HDGC have a germline mutation in the tumor suppressor gene E-cadherin or CDH1. The inactivation of the second allele of E-cadherin through mutation, methylation, and loss of heterozygosity eventually triggers the development of gastric cancer. In the familial syndrome hereditary diffuse gastric carcinoma (HDGC), diffuse gastric carcinoma and occasionally infiltrating lobular carcinoma of the breast develop in young adults. Germline mutations in the CDH-1/E-cadherin gene are, to date, the only known cause of hereditary diffuse gastric cancer (HDGC). E-cadherin is a member of the cadherin family of homophilic cell adhesion proteins that are central to the processes of development, cell differentiation, and maintenance of epithelial architecture. It is the predominant cadherin family member expressed in epithelial tissue and is localised at the adherens junctions on the basolateral surface of the cell. E-cadherin is downregulated in a broad range of epithelial tumours and its loss is associated with an infiltrating phenotype and poor prognosis.1,3,5,7,10 Recently, E-cadherin gene (CDH1) germ-line mutations have also been identified as the underlying genetic basis for another familial gastric cancer syndrome that is characterized by early occurrence of diffuse type adenocarcinoma. These patients are also at risk for developing lobular breast cancer. Sporadic gastric carcinomas, especially diffuse carcinomas, exhibit reduced or abnormal E-cadherin expression, and genetic abnormalities of the E-cadherin gene and its transcripts. Reduced E-cadherin expression is associated with reduced survival. Somatic E-cadherin gene alterations also affect the diffuse component of mixed tumours. Alpha-catenin, which binds to the intracellular domain of E-cadherin and links it to actin-based cytoskeletal elements, shows reduced immunohistochemical expression in many tumours and correlates with infiltrative growth and poor differentiation. Beta catenin may also be abnormal in gastric carcinoma. 1,2,5,6,7,10 Clinical features, gastric cancers that elicit symptoms are usually late-stage tumors and are more likely to precede the diagnosis of cancer in Western, unscreened populations than in Asia or other high-risk areas. The early cancers that may accompany chronic gastric ulcers are exceptions to this rule. A review of 18,265 stomach cancers by the American College of Surgeons found the following frequently overlapping symptoms to be the most common: Weight loss (61.6%), abdominal pain (51.6%), nausea (34.3%), dysphagia (26.1%), and melena (20.2%). Weight loss is an ominous presenting symptom and associates with poor survival. Early satiety may result from either pyloric obstruction or the ability of the stomach to expand. Blood loss from an ulcerated tumor may induce anemia and fatigue. Infection of necrotic, fungating tumors may cause fever. Spread of gastric cancer to a distant site is the presenting symptom in some cases. This may take the form of an enlarged supraclavicular lymph node. Ascites or vaginal bleeding due to endometrial metastases may be the presenting symptom in premenopausal women with diffuse cancer.1,2Lymphatic spread occurs early, and as noted above, may even be observed with small submucosal cancers. Lymphatic and vascular invasion carries a poor prognosis and is frequently seen in advanced cases. Surgical resection margins should be monitored by frozen section because gross assessment is inaccurate and margin involvement occurs in 15% of cases. The pathologist should take pains to identify the proximity of the cancer to the radial margin, which is defined as the surgically dissected surface adjacent to the deepest point of tumor invasion. A deeply penetrating cancer that centers on the lesser or greater curvature may have a radial margin that lies in the connective tissue of the lesser or greater omentum, rather than the serosal surface. Microscopic involvement of a resection margin is almost synonymous with early recurrence or death.2Treatment, surgical removal of stomach cancer is the treatment of choice, although, if you say the cancer has spread, an operation to remove the cancer is unlikely to be of benefit. Several clinical studies have reported results that indicate a moderate survival or palliative benefit for patients with advanced stomach cancer. There is no combination of chemotherapy which is clearly superior to others, but most active regimens include 5-Fluorouracil (5-FU), Cisplatin, and/or Etoposide. The combination of 5-Fluorouracil, doxorubicin, and mitomycin (FAM) has been used in the past with modest success. There was no statistically significant difference in survival among the various chemotherapy combinations on their preliminary report.9 The almost limitless heterogeneity of most advanced gastric cancers creates a barrier to the development of customized chemotherapeutic approaches. Palliation, rather than cure, is the primary goal of adjuvant therapy. Most patients who have had a resection for gastric cancer with extensive lymph node metastases will have a disease relapse. No randomized trial has shown a benefit for this subset of patients and no single agent has proved effective for postoperative chemotherapy. This is not unexpected since successful response to treatment with drugs that target specific oncogenes allows subsets of other cells to survive and serve as the seedbeds for recurrent tumors. 2,4 As far as diet, nitritional support of stomach cancer is a very important component of the treatment. Many patients are malnourished with some extent of weight loss prior to their diagnosis. If he is able to eat, well balanced diet with adequate protein and vegetables is appropriate.9Prognosis and predictive factors, when early gastric cancer is confined to the mucosa or is node negative, the reported five year survival is now over 90% in almost all Western and Japanese series.7 Lymphatic and vascular invasion carries a poor prognosis and is often seen in advanced cases and it is also an important prognostic indicator. Patients who had nodal involvement in 1-6 lymph nodes (pN1), the 5-year survival rate was 44% compared with a 30% survival rate in patients with 7-15 lymph nodes involved with tumour (pN2). Patients with more than 15 lymph nodes involved by metastatic tumour (pN3) had an even worse 5-year survival of 11%. Gastric carcinoma with obvious invasion beyond the pyloric ring, those with invasion up to the pyloric ring, and those without evidence of duodenal invasion have 5-year survival rates of 8%, 22%, and 58%, respectively.m. Patients with T1 cancers limited to the mucosa and submucosa have a 5-year survival of approximately 95%. Tumours that invade the muscularis propria have a 60-80% 5-year survival, whereas tumours invading the subserosa have a 50% 5-year survival. Unfortunately, most patients with advanced carcinoma already have lymph node metastases at the time of diagnosis.1,5Histological features. The value of the histological type of tumour in predicting tumour prognosis is more controversial. The overall survival rate of patients with signet ring cell carcinoma was worse than that of patients with other types of gastric cancer. The late stage of signet ring cell carcina could not axplain this difference. Because of the frequency of peritoneal dissemination, strong consideration should be given to evaluating the role of intraperitoneal chemotherapy. The recent study also revealed that early signet ring cell carconama of the stomach is not lethal as was previously beleived (Tso et al. 1987).1,4Cytologic subtyping of gastric cancer is not essential. If one equates papillary, tubular, and some mucinous carcinomas with Lauren's intestinal-type and signet ring cell carcinoma with the diffuse type, then a specificity rate for cytologic typing of >90% for the former and of 85% for the latter may be obtained. However, vacuolized cells should be evaluated with special care because signet ring cells are easily simulated by goblet cells. The size of the nucleolus and the nuclear outline are helpful differential diagnostic features. Unless fine needle aspiration is combined with the endoscopic examination, early gastric carcinoma cannot be identified cytologically because no features exist that allow one to distinguish between early and advanced gastric cancer. 2Machara et al, 1992 reported that patients with signet ring cell carcinoma can expect a longer survival than those with other types of gastric cancer. In contrast, Kim et al, 1994 reported that there was no significant difference in the 5-year survival rates between patients with early signet ring cell type and those with other types of advanced cancer. Multivariate analysis showed the significant prognostic factors to be vascular invasion and tumor location. Microinvasion has been reported tobe a major independent risk factor for long-term survival (Yokota et al. 1998). Microinvasion may represent an early finding of metastatic spread of gastrointestinal tumors, and capillary microinvasion could predispose to distant metastasis. The tumor location was also an important prognostic variable, and cancer that had invaded the whole stomach was worse than that for patients with cancer that had invaded only the antrum and body of the stomach. 4
References
1. Hamilton S R, Aaltonen L A. Pathology and Genetics of Tumours of the Digestive System.Tumours of the Stomach. IARC Press. Lyon, 2000. p.35-50.
2. Preiser F. Cecilia M, Noffsinger, Amy E. The Neoplastic Stomach. In: Gastrointestinal Pathology: An Atlas and Text, 3rd Edition. Lippincott Williams & Wilkins. 2008.p.234-70
3. Henson DE, Dittus C, Younes M, et al: Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, Arch Pathol Lab Med 2004;128:765.
4. Yokota T, Kunii Y, Teshima S, et al. Signet Ring Cell Carcinoma of the Stomach: A Clinicopathological Comparison with the Other Histological Types. Available from: www.journal.med.tohoku.ac.jp.pdf. 20/1/2014. 01.30
5. Hall C R. Pathology of Gastric cancer. In: Gore R M. Reznec R H. Gastric Cancer. Contemporary Issues in Cancer Imaging. A Multidisciplinary Approach. Cambridge University Press. 2010.p.22-42.
6. Lin C, Crawford J M. The Gastrointestinal Tract. In: Kumar V, Abbas A K, Fausto N. Robbin and Cotran Pathologic Basis of Disease. Seventh Edition. Elsevier Saunders. 2005.p.871-.
7. Lee S S, Ryu S W, Kim I H et al. Early Gastric Cancer with Signet Ring cell Histology Remained Unresected for 53 Moths. Available from: www.ncbi.nlm.nih.gov/pmc. on February 21, 2014. 15:37.
8. Hereditary diffuse gastric cancer: predominance of multiple foci of signet ring cell carcinoma in distal stomach and transitional zone. Available from: gut.bmj.com . On February 20, 2014. 14:52.
9. Liu L. Signet Ring Cell Cancer. Available from: www.oncolink.org. On February 2, 2014. 04:26.
10. Hu B, Hajj N E, Sittler S et al. Gastric cancer: Classification, histology and application of molecular pathology. Available from: http://www.thejgo.org/article/view/427/html On February 2, 2014. 04:05.















How I became a happy woman again
BalasHapusWith tears of joy and happiness i am giving out my testimony to all viewers on line, my problem with Stomach Cancer stage IB has caused me many pains and sadness especially in my family.
I was so afraid of loosing my life, I suffered the embarrassment of visiting
therapy hundreds of times, unfortunately they did not find a definitive solution to my problem, I cried all day and night, do I have to live my life this way? I searched all true the internet for care, I was scammed by internet fraudsters times without numbers… until a friend of mine who stays in the UK introduced me to a friend of hers who was cured of the same disease, and she introduced me to Dr Itua who cured her from Breast Cancer by this email/WhatsApp +2348149277967, drituaherbalcenter@gmail.com. I contacted him and he promised that all will be fine and I had faith.He send me his herbnal medicines through Courier servcie and i was instructed on how to drink it for three weeks to cure,I followed the instruction given to me and Today am a happy woman again. He cures all kind of diseases like__Brain cancer,Gestational trophoblastic disease,Head and neck cancer,Ovarian cancer,Hodgkin lymphoma,Herpes,,Liver cancer,Throat cancer,
Syndrome Fibrodysplasia Ossificans ProgresSclerosis,Alzheimer's disease,Chronic Diarrhea,Copd,Parkinson,Als,Adrenocortical carcinoma Infectious mononucleosis.
Intestinal cancer,Thyroid Cancer,Uterine cancer,Fibroid,Angiopathy, Ataxia,Arthritis,Amyotrophic Lateral Scoliosis,Brain Tumor,Fibromyalgia,Fluoroquinolone ToxicityBladder cancer,Hiv,Esophageal cancer,Gallbladder cancer,Kidney cancer,Hpv,Lung cancer,Melanoma,Mesothelioma,Multiple myeloma,Neuroendocrine tumors
Non-Hodgkin lymphoma,Oral cancer,Sinus cancer,Hepatitis A,B/C,Skin cancer,Soft tissue sarcoma,Spinal cancer,Stomach cancer,Vaginal cancer,Vulvar cancer,
Testicular cancer,Tach Diseases,Leukemia.
This is an insightful and important discussion on the risks of relying solely on surrogate outcomes in clinical trials. The case of Rilotumumab highlights how crucial it is to evaluate Gastric Cancer Drugs based on real patient outcomes, not just biomarkers. Ensuring safety and long-term survival should always come before promising early lab results.
BalasHapus